Injectable (Syringe Pro) - Automated Syringe Filling Solutions
Benefits of pre-filled syringes
A growing number of drugs and vaccines are available in prefilled syringes. The manufacture and supply of prefilled syringe products is highly regulated to minimise chemical and physical changes and maintain drug efficacy. Compared to oral administration, parenteral drug delivery provides rapid drug absorbed and avoids first-pass metabolism, or enzymatic degradation. This leads to better bioavailability, and more predictable pharmaco-dynamic and pharmacokinetic profiles. Compared to traditional parenteral delivery, prefilled syringes are easy to use, eliminate dosing errors and are not wastefully overfilled.

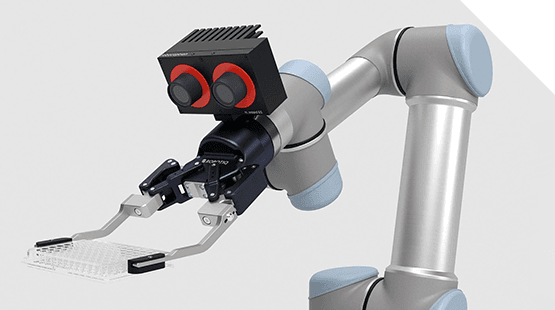
Automate syringe testing with SyringePro
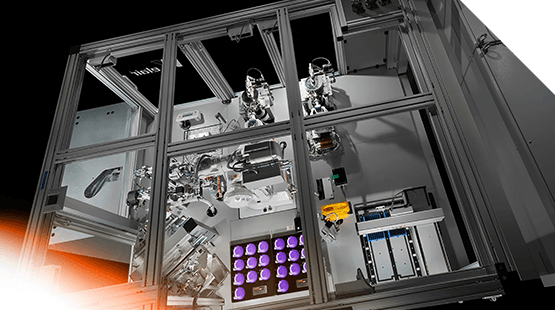
Testing is an important step in the manufacturing process for prefilled syringes to ensure product quality and stability. Patient safety, drug stability, and ease of filling are being addressed with new designs and coatings and materials. Astech’s Syringe Pro system is designed to provide safe, high throughput, testing of prefilled syringe products for the highly regulated parenteral drug market.

Equipment
Examples of the type of equipment included within the system are as follows:
Device Loading Tray Area
Syringe Carousel
Syringe Sheath Removal Station
Syringe Deposit Station
Solvent Dispense Station
Automated needle/tip wash
Vortexer
HPLC interface
Liquid Handling System (includes HPLC injection valve)

Software
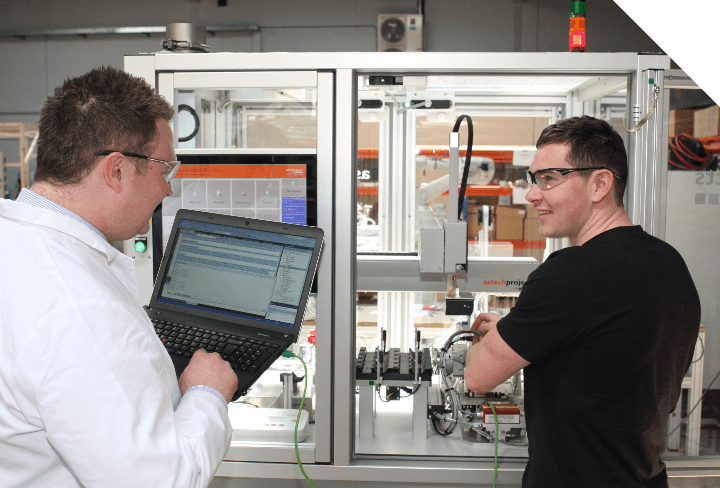
The system is fully enclosed with the option of including environmental control or extraction. Syringe Pro® systems can be controlled by a modular software solution based around Astech’s proven architecture. This software solution is designed to run syringe test methods specific to your requirements, and can include compliance to 21 CFR Part 11 if required. Operators simply load the system with a batch of prefilled syringes from the production line and select the required test protocol for each syringe via the GUI. The system automatically performs testing and report analysis results back to the database. The main components of the software system are as follows:
Control Server
Database Schema
Graphical User Interface (GUI)

Discover more about Astech
Explore our Respiratory Applications, Products plus REACTS
Get in contact with us
![[object Object]](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2Fpk0repno%2Fproduction%2Fdbf3a5f83007da9e59edb68fb82b530f003f1265-376x220.png&w=828&q=75)

![[object Object]](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2Fpk0repno%2Fproduction%2F1129b34fb570be1e0a58285546ffbcba387248c2-838x342.png&w=1920&q=75)