Automated recovery of samples from NGI cup trays
Aerosol and dry powder inhalers
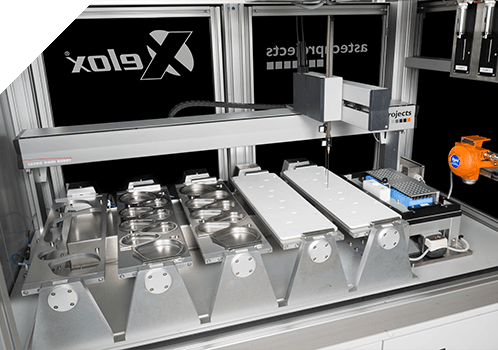
Aerosol and Dry Powder inhalers that contain drug, either in a reservoir or as pre-metered dosage, should be subjected to a test designed to measure Aerodynamic Particle Size Distribution (APSD). This should be measured over the entire inhaler contents. For many companies, recovery of drug sample from NGI cup trays is still performed partially or completely manually. Automating this process can increase throughput, reduce risk of repetitive strain injury for laboratory personnel, reduce variability of results and improved availability. Automated recovery of samples from NGIs is possible with the Xelox® Series NGI Sample Recovery System.

Automating Inhaler NGI testing is possible with the Xelox® 1 Series NGI Sample Recovery System
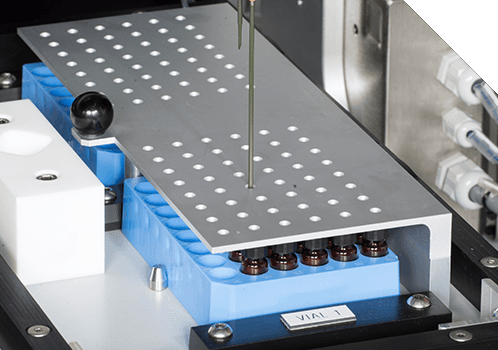
The Xelox® NGI Sample Recovery System (SRS) can process up to 5 separate NGI trays. The system automates the otherwise manual process of sample dispensing, agitating and recovery from NGI equipment. The NGI SRS dispenses a pre-metered volume of solvent into each NGI cup tray, using an automated pipette. The tray assembly is then agitated to fully dissolve the drug captured on each NGI cup tray. Upon completion of the agitation sequence, a small sample of homogenised drug solution is withdrawn, individually from each NGI cup and dispensed into sample vials. The finished vialled samples are then ready for HPLC/UPLC analysis, to determine the amount of drug contained in each sample recovered.
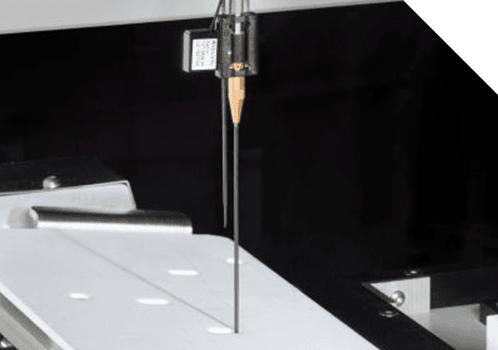
Core Functions
Parallel processing and ‘Just in Time’ software scheduling to optimise throughputs and process 5 trays in less than 60 minutes
Configurable stage recovery order/sequencing gives you control
High precision syringe pumps can handle very small volumes
Configurable motion profiles for agitation (angle, duration and frequency) – optimise solute dissolution for faster processing time
Intuitive operator interface with touchscreen controls – simple and easy to use with password protection and access levels for all types of users
Email notification system to inform operators that runs are complete – don’t be chained to your equipment
Ethernet based control system with no requirement for third party licensing and/or fieldbuses – doesn’t require any special modifications to your IT infrastructure
Core Functions
Waste solvent capacity for 1 week of running, in fire-rated cabinets
Small system footprint and minimal effect on lab conditions – no special modifications to infrastructure needed to install the system
Built-in extraction system (+ monitoring) – no additional equipment needed
Modular design components and expandable control system – build your full automation solution as needed
Remote diagnostics support functions via remote access tools or VPN – many queries can be resolved without the need for an onsite engineer
Long term retention of sample tracking data assists with 21 CFR Part 11 compliance
LIMS compatible data exporting functions, configurable report templates and system performance tracking.

Customise
The intuitive user interface lets you easily select methods and run programs. The software allows full traceability and control over access for compliance and auditing. The throughput of the system is dependent on procedure and parameters selected in the method and hardware configuration. However, typically over an 8-hour shift, using a method with a 10ml solvent dispense volume and a 10-minute agitation Time, the system will be capable of processing a total of 60 tray assemblies. The NGI SRS can be integrated with other Astech Projects X- Products systems to provide complete automation of inhaled device drug testing.
Get in contact with us
![[object Object]](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2Fpk0repno%2Fproduction%2Fdbf3a5f83007da9e59edb68fb82b530f003f1265-376x220.png&w=828&q=75)

![[object Object]](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2Fpk0repno%2Fproduction%2F1129b34fb570be1e0a58285546ffbcba387248c2-838x342.png&w=1920&q=75)