Xelair® Dose Content Uniformity of the Emitted Dose
Key benefits of Xelair® Automation
Reduced variability in results
Increased volumes of test data
Increased productivity
Contained handling of drug product, minimising operator exposure
Upgrade paths throughout the Xelair® range
Significant reduction in Health and Safety issues including WR-ULD
Harmonised testing throughout the Xelair® range
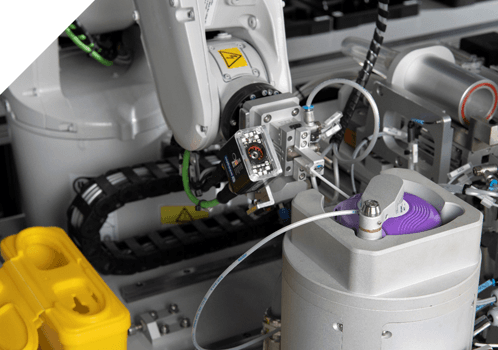
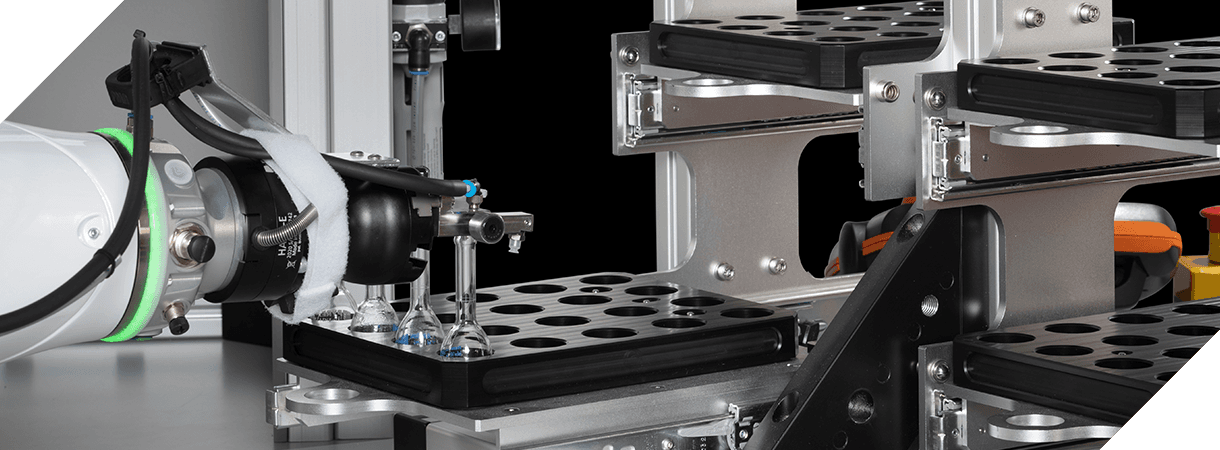
Xelair® 1, 3 and 5 Series
Xelair® 1 Series The Xelair® 1 Series are analyst workstations. They automate individual stages of dose content uniformity testing. Xelair® 3 Series The Xelair® 3 Series are semi-automated platforms. They automate the entire through life testing of an inhaler device. Xelair® 5 Series The Xelair® 5 Series are fully automated systems. They offer 24/7 unattended operation.
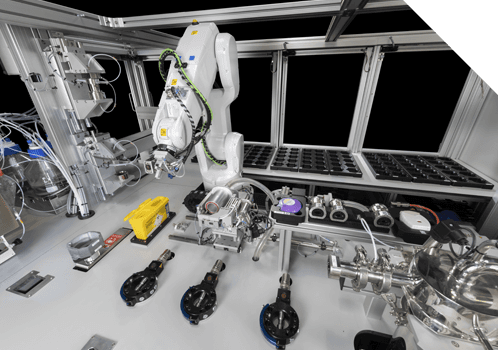
DCU Evaluation kit
Manual test apparatus is available for evaluation, method development and routine laboratory use on a commercial loan or purchase basis. The apparatus uses the core technology at the heart of the Xelair® range. Methods developed using the manual apparatus can be transferred onto any platform in the Xelair® range. Uses Astech core technology for dose collection and dose recovery operations.
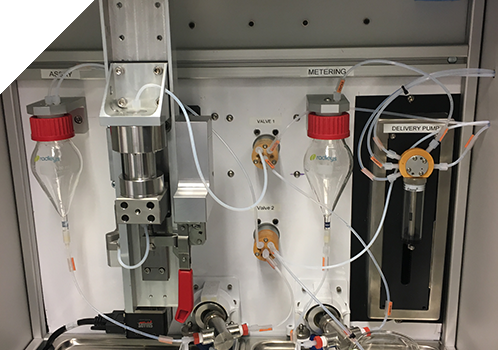
UoDD, DDU or DCU?
Within the safety and efficacy testing of inhaled drugs the terminology Uniformity of Delivered Dose, Delivered Dose Uniformity or Dose Content Uniformity are used depending on the pharmacopoeia. Whilst broadly similar, a differing sequence of sampling is required to meet the guidelines. Astech’s Relovex® software allows simple set up of waste fire and sample collection profiles. Additionally, Relovex® provides a fully traceable and 21CFR Part11 compliant digital record.
Get in contact with us
![[object Object]](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2Fpk0repno%2Fproduction%2Fdbf3a5f83007da9e59edb68fb82b530f003f1265-376x220.png&w=828&q=75)
![[object Object]](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2Fpk0repno%2Fproduction%2F1129b34fb570be1e0a58285546ffbcba387248c2-838x342.png&w=1920&q=75)